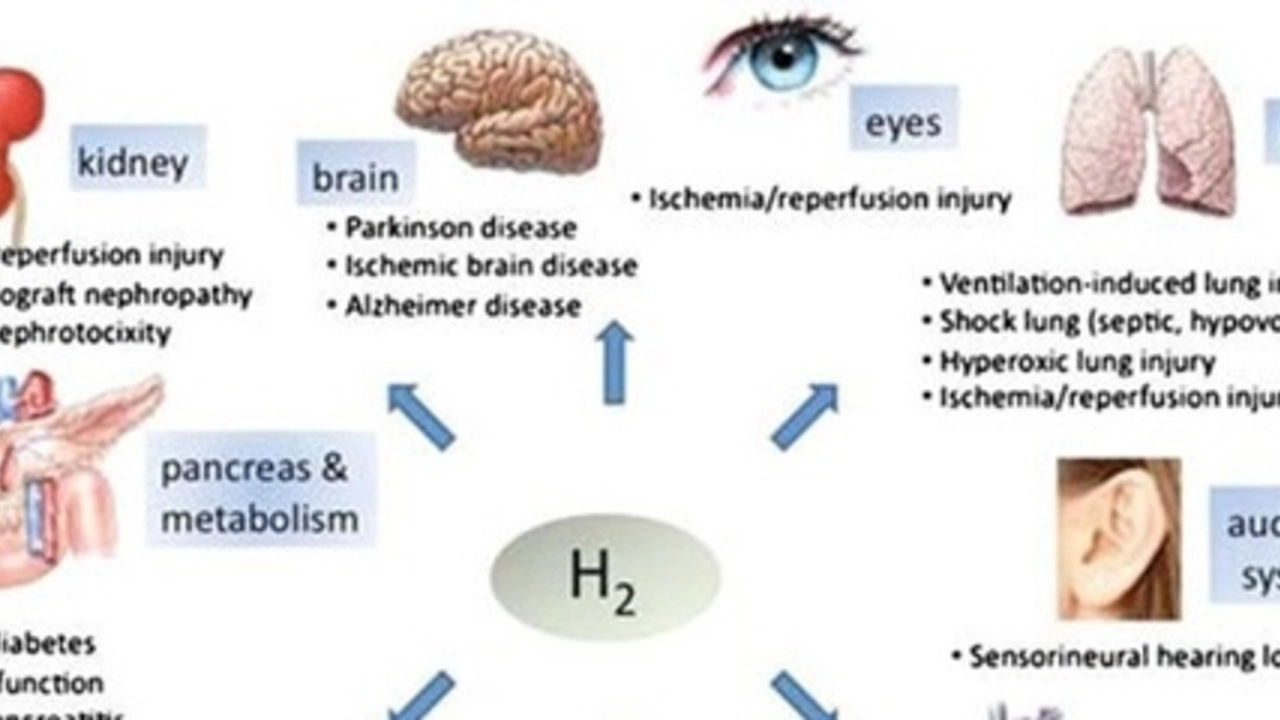
Understanding Reperfusion Injury
Let's start off by getting clear on what reperfusion injury is. In simple terms, reperfusion injury is the tissue damage caused when blood supply returns to the tissue after a period of ischemia or lack of oxygen. The reoxygenation results in inflammation and oxidative damage through the induction of oxidative stress rather than the restoration of normal function. This kind of injury is often seen in instances such as stroke, heart attack, and organ transplants.
It might seem counterintuitive at first - shouldn't the restoration of blood flow be a good thing? Well, while it is crucial to restore blood flow, the sudden return can cause serious damage. This is because the process can lead to a series of harmful reactions, including inflammation and cell death. So, it's a bit of a double-edged sword.
The Mechanism of Autoimmune Disorders
Now let's shift our focus to autoimmune disorders. These conditions occur when your immune system mistakes your body's own cells as foreign invaders and starts attacking them. From rheumatoid arthritis to lupus and type 1 diabetes, there's a wide range of autoimmune disorders, each affecting different parts of the body.
While the exact cause of these disorders is still unclear, it’s believed that a combination of genetic, environmental and hormonal factors play a role. In these conditions, the immune system fails to differentiate between self and non-self, leading to an immune response against its own cells and tissues. The symptoms can vary widely, depending on which part of the body is being attacked.
Linking Reperfusion Injury with Autoimmune Disorders
At first glance, reperfusion injury and autoimmune disorders may seem like two entirely separate medical issues. However, recent research has suggested a potential link between the two. This connection can be traced back to the immune response triggered during reperfusion injury.
When tissues are damaged due to reperfusion injury, the body's immune response isn't just restricted to the affected area. The immune cells that get activated during this process can circulate through the body, potentially triggering an autoimmune response. This could explain why some individuals develop autoimmune disorders after experiencing a reperfusion injury.
Unfolding the Role of the Immune System
The immune system plays a pivotal role in both reperfusion injury and autoimmune disorders. In the case of reperfusion injury, the immune system gets triggered as it tries to repair the damaged tissue. However, this immune response can inadvertently lead to additional tissue damage.
Similarly, in autoimmune disorders, the immune system gets confused and starts attacking the body's own cells. This misguided immune response can lead to a variety of symptoms, depending on which part of the body is affected. So, while the immune system is meant to protect us, in these cases, it can end up causing harm.
Exploring Potential Treatments
Understanding the connection between reperfusion injury and autoimmune disorders can open new avenues for treatment. For instance, therapies that target the immune system could potentially be beneficial for both conditions.
Anti-inflammatory drugs, immunosuppressants, and antioxidants are currently being explored as potential treatments for reperfusion injury. Similarly, treatments for autoimmune disorders often involve suppressing or modulating the immune system to reduce the harmful attacks on the body's own cells.
Future Directions in Research
There's still much we don't know about the connection between reperfusion injury and autoimmune disorders. However, the potential link between the two offers promising avenues for future research.
By deepening our understanding of the immune response in reperfusion injury, we could potentially develop new strategies to prevent or treat autoimmune disorders. And similarly, insights from autoimmune research could also shed light on ways to mitigate the harm caused by reperfusion injury. Ultimately, our goal is to improve the quality of life for patients affected by these conditions.


Dean Gill
July 6, 2023 AT 20:45Reperfusion injury, despite being a necessary physiological response, triggers a cascade of events that can paradoxically worsen tissue outcomes. When blood rushes back into ischemic tissue, the sudden surge of oxygen fuels the production of reactive oxygen species, which in turn damage cellular membranes and DNA. The injured cells release danger-associated molecular patterns, alerting resident immune cells and recruiting neutrophils from the circulation. These neutrophils unleash proteases and additional oxidants, amplifying the local inflammation and creating a hostile microenvironment for recovering cells. Moreover, the endothelial lining of blood vessels becomes permeable, allowing plasma proteins and inflammatory mediators to spill into the interstitial space. This leakage not only hampers nutrient delivery but also exposes antigens that were previously hidden from immune surveillance. As a result, the immune system may begin to recognize self‑components as foreign, setting the stage for an autoimmune response. Clinical observations have noted that patients who experience severe reperfusion injury after myocardial infarction or stroke sometimes develop autoantibodies targeting cardiac or neural tissue. Experimental models corroborate this, showing that blocking specific cytokines during reperfusion can reduce the subsequent development of autoimmunity. From a therapeutic perspective, this connection opens a window for interventions that target both oxidative stress and immune modulation simultaneously. Antioxidant therapies, such as N‑acetylcysteine, have shown mixed results, but combining them with selective immunosuppressants may yield synergistic benefits. In addition, pre‑conditioning strategies that gently expose tissue to brief periods of ischemia can prime the immune system to respond more calmly when full reperfusion occurs. Finally, personalized medicine approaches that assess a patient’s genetic predisposition to autoimmunity could inform the choice of post‑reperfusion treatments, minimizing the risk of chronic immune‑mediated disease. While the research is still evolving, the emerging evidence underscores the importance of viewing reperfusion injury not just as a local event, but as a potential catalyst for systemic immune dysregulation.
Royberto Spencer
July 6, 2023 AT 21:00One might argue that the very act of restoring flow is a moral imperative, yet the universe often reminds us that good intentions can bear unintended consequences. The paradox of reperfusion mirrors the ancient philosophical dilemma of the "double‑edge sword," where the same force that heals may also wound. It is not merely a biochemical mishap; it is a reflection of the fragile balance between order and chaos inherent in living systems. When we intervene, we must ask whether we are merely accelerating an inevitable destiny or reshaping it with responsibility.
Annette van Dijk-Leek
July 6, 2023 AT 21:16Wow!!! This whole thing is fascinating, and it really shows how resilient our bodies can be!! But also, wow, the immune system can be such a drama queen when it gets over‑excited!!! Keep pushing forward, researchers, you’re doing amazing work!!! 😊😊😊
Katherine M
July 6, 2023 AT 21:33Esteemed colleagues, the discourse surrounding the immunological sequelae of reperfusion injury warrants a nuanced and culturally cognizant approach. One must consider the historical narratives that have shaped our understanding of autoimmunity, as well as the diverse patient populations affected by these phenomena. It is with great respect that I invite further scholarly exchange on this matter. 🤝📚
Bernard Leach
July 6, 2023 AT 21:50Reperfusion brings oxygen back to starving cells. This oxygen fuels free radicals. Free radicals damage cell membranes. Damaged cells release signals. Signals attract immune cells. Immune cells release enzymes. Enzymes cause more damage. The cycle continues. The body’s repair mechanisms become confused. This confusion can lead to auto‑reactivity. Auto‑reactivity manifests as autoimmune disease. Preventing this requires early intervention. Antioxidants help reduce free radicals. Immunomodulators can calm the immune response. Combining both may offer the best outcome.
Shelby Larson
July 6, 2023 AT 22:06Obviously the whole thing is just a textbook case of overblown hype.
Mark Eaton
July 6, 2023 AT 22:23It’s encouraging to see how many therapeutic angles are being explored! Targeting oxidative stress while also modulating the immune response could really tip the scales toward recovery. Keep sharing updates, the community thrives on this momentum.
Alfred Benton
July 6, 2023 AT 22:40The mainstream narrative hides the fact that large pharma suppresses true cures in favor of profit.
Susan Cobb
July 6, 2023 AT 22:56While it’s tempting to buy into grand conspiracies, the data actually suggest that the modest benefits observed with antioxidant adjuncts are statistically insignificant. A balanced evaluation of the literature is essential before drawing dramatic conclusions.
Ivy Himnika
July 6, 2023 AT 23:13Just a quick note: the term "reperfusion injury" should be hyphenated as "re‑perfusion injury" for clarity. Also, remember to use the Oxford comma in lists – it improves readability. 👍😊
Nicole Tillman
July 6, 2023 AT 23:30Considering the evidence, it is clear that a multidisciplinary approach-combining antioxidants, precise immunosuppression, and patient‑specific risk profiling-offers the most pragmatic path forward. Let’s focus our efforts on collaborative trials that respect both scientific rigor and patient safety.