When a generic drug hits the market, it doesn’t mean the work is done. In fact, the real challenge often begins after approval. Generic manufacturers aren’t allowed to just tweak their processes, switch suppliers, or upgrade equipment without telling the FDA-and sometimes, they need to stop everything and wait for approval. The question isn’t whether a change can be made, but what triggers re-evaluation.
Not All Changes Are Created Equal
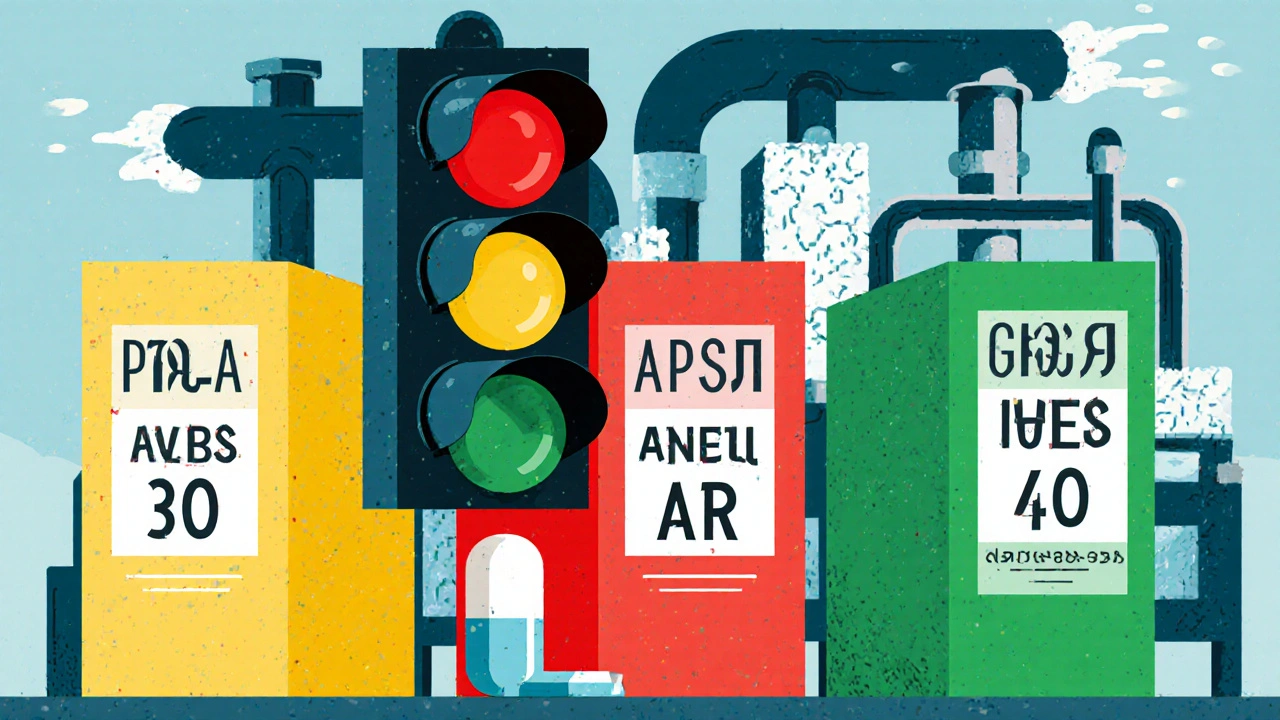
The FDA doesn’t treat every manufacturing change the same way. Think of it like a traffic light system: green, yellow, red. Minor tweaks? Green light. Bigger changes? Yellow light-notify the FDA and proceed while waiting. Major changes? Red light-stop, submit, and wait for approval before making the switch. These categories are officially called:- Prior Approval Supplements (PAS): These are the red lights. You can’t make the change until the FDA says yes. This applies to changes that could affect the drug’s safety, effectiveness, or quality-like switching to a new active ingredient supplier, changing the synthesis route, or moving production to a completely new factory.
- Changes Being Effected (CBE): These are the yellow lights. You can make the change immediately but must notify the FDA within 30 days (CBE-30) or immediately (CBE-0). This includes things like tightening a specification or updating packaging materials, as long as you can prove it won’t impact the drug.
- Annual Reports (AR): These are the green lights. You make the change, document it, and report it once a year. Think minor equipment swaps or small adjustments to process parameters that have been validated over time.
Here’s the kicker: if you guess wrong and make a PAS-level change without approval, the FDA can pull your product off the market. That’s not just a delay-it’s a financial hit that can cost millions.
What Specifically Triggers a Full Re-Evaluation?
Not every change needs a PAS, but these are the big ones that almost always do:- Changing the drug substance manufacturer: If you switch from one API supplier to another, especially if they use a different synthesis method, the FDA needs to verify the new version is identical in purity, impurity profile, and performance. Even a 0.1% difference in an impurity can trigger a full review.
- Scale-up or scale-down: Making 10,000 tablets a day? Fine. Jumping to 1 million? That’s not just bigger-it’s different. Equipment, mixing times, heat transfer, and drying rates all change. The FDA requires full process validation and stability data for at least six months.
- Changing the manufacturing site: Moving from a plant in India to one in Ohio isn’t just logistics-it’s a whole new set of controls, personnel, and environmental factors. Facility transfers are among the most common triggers for PAS submissions.
- Modifying the formulation: Even swapping one inactive ingredient (like a binder or coating) can alter how the drug dissolves. For generics, bioequivalence to the brand-name drug is everything. If the dissolution profile changes, you need new clinical data.
- Introducing new technology: Moving from batch manufacturing to continuous manufacturing? That’s a game-changer. While the FDA supports innovation, it also requires proof that the new process consistently produces the same product. Teva’s shift to continuous manufacturing for amlodipine took 8 months to approve-thanks to pre-submission meetings and detailed data.
A 2022 case study showed a company that increased batch size by 30% for a tablet needed 6 months of stability data, 12 months of process validation, and a 14-month FDA review. That’s over a year of lost production time-just to make a change that should’ve improved efficiency.
Why Does This Take So Long?
The average PAS review takes 10 months. That’s not because the FDA is slow-it’s because they’re thorough. They’re not just checking paperwork. They’re looking at:- Comparative analytical data: Is the new product chemically identical to the old one?
- Stability data: Does it still last 24 months on the shelf?
- Process validation: Can you prove you can make this consistently?
- Facility inspection readiness: Is the site clean, compliant, and documented?
And here’s the reality: 68.4% of PAS submissions get a complete response letter-meaning the FDA asks for more data. The most common reasons? Analytical method changes (28.7%), facility transfers (24.5%), and formulation tweaks (19.3%).
Small manufacturers suffer the most. Companies with fewer than five ANDAs report review times 43% longer than big players. Why? They don’t have dedicated regulatory teams or the budget for multiple pre-submission meetings with the FDA. One quality assurance professional on Reddit described waiting 18 months for approval to upgrade a tablet press-even though the final product met all specs. The FDA needed more documentation on process control parameters. No one knew exactly what they needed until they got the letter.
How Companies Are Fighting Back
The system is broken for many. The average PAS costs $287,500 to submit. For a generic drug that sells for pennies, that’s not an investment-it’s a gamble.But smart companies are changing the game:
- Quality by Design (QbD): Instead of building a product and testing it, they design the process to be robust from the start. By understanding how variables like temperature, pressure, and mixing speed affect the final product, they build in flexibility. This can reduce post-approval changes by up to 40%, according to former FDA director Dr. Jane Smith.
- Process Analytical Technology (PAT): Real-time monitoring of production lets companies catch deviations before they become problems. Companies using PAT saw 32.6% fewer PAS submissions over five years.
- Pre-submission meetings: Talking to the FDA before you submit saves time. Teva’s amlodipine approval took 8 months instead of 14 because they met with regulators early and aligned on data expectations.
There’s also a new incentive: the ANDA Prioritization Pilot Program, launched in September 2023. If your generic drug is made entirely in the U.S.-from active ingredient to finished tablet-you can get reviewed in 8 months instead of 30. The FDA is betting that faster approvals will lure manufacturers back to U.S. soil. By 2026, nearly 40% of new generics could qualify.
The Bigger Picture: Why This Matters
This isn’t just about paperwork. It’s about drug supply chains. When a manufacturer in Europe shuts down a line due to a failed inspection, and the U.S. can’t quickly approve a replacement, patients go without. That’s why the FDA is pushing for domestic manufacturing and better processes.But there’s a catch. Many manufacturers avoid making improvements because the risk outweighs the reward. Why upgrade to a more sustainable, efficient process if it means a year-long pause and $300,000 in fees?
That’s changing. The FDA’s draft guidance for complex generics (released January 2024) proposes a tiered risk system that could cut PAS submissions by 35% for minor changes. The PreCheck program for facilities could cut approval time in half. And with GDUFA IV negotiations happening in 2025, the rules might get clearer, fairer, and faster.
For generic drug makers, the lesson is simple: don’t wait until you’re forced to change. Build flexibility into your process now. Document everything. Talk to the FDA early. And remember-the goal isn’t just to get approved. It’s to stay approved, without interruption, for years to come.
What Happens If You Don’t Report a Change?
Skipping a PAS or misclassifying a change isn’t just a paperwork error-it’s a violation. The FDA can issue a warning letter, seize inventory, or even block future applications. In 2021, a generic manufacturer was fined $2.3 million for making a formulation change without approval. The product was pulled from shelves. Patients were affected. The company’s reputation took years to recover.There’s no gray area here. If you’re unsure whether a change requires a PAS, assume it does. Consult your regulatory team. Reach out to the FDA. It’s cheaper than getting shut down.
What’s the difference between a PAS and a CBE-30?
A Prior Approval Supplement (PAS) requires FDA approval before you can make the change. You can’t implement it until they say yes. A CBE-30 lets you make the change immediately, but you must notify the FDA within 30 days. PAS is for high-risk changes like new manufacturing sites or major process shifts. CBE-30 is for lower-risk changes like tightening specifications or updating labeling.
Can I change my drug’s packaging without FDA approval?
You can change packaging materials (like switching from glass to plastic bottles) as long as it doesn’t affect the drug’s stability or safety. That’s usually an Annual Report. But if you change the shape, color, or markings in a way that could confuse patients or affect compliance, you may need a CBE-30. Always test for compatibility-some plastics can leach chemicals into the drug.
Why do facility transfers take so long to approve?
Transferring production to a new site means the FDA must inspect it, verify that all processes work the same, and confirm the product is still bioequivalent. Even if the equipment is identical, differences in humidity, operator training, or even the water supply can affect the outcome. The FDA requires full validation data, stability studies, and often a pre-approval inspection-which can take months to schedule.
Are there any shortcuts for small manufacturers?
Yes. The FDA’s PreCheck program and the ANDA Prioritization Pilot can speed things up if you manufacture in the U.S. and source your active ingredient domestically. Also, joining the Generic Drug User Fee Amendments (GDUFA) program gives you access to dedicated review teams and pre-submission meetings. Small companies often miss these opportunities because they don’t know they exist.
How do I know if my change is low-risk?
Start with the FDA’s 2011 guidance on supplements. It lists examples of changes that fall under each category. If your change isn’t listed, compare your product’s analytical data before and after the change. If everything matches within tight limits (like impurity profiles, dissolution curves, and particle size), it’s likely low-risk. When in doubt, consult a regulatory expert or request a pre-submission meeting with the FDA.
What Should You Do Next?
If you’re a generic manufacturer:- Review your current manufacturing process. What changes have you made since approval?
- Map each change to the FDA’s categories. Are any of them PAS-level?
- If you’re planning a change-don’t start yet. Schedule a pre-submission meeting with the FDA.
- Invest in Quality by Design and real-time monitoring tools. They’ll save you time and money in the long run.
- Consider applying for the ANDA Prioritization Pilot if you’re U.S.-based.
For everyone else-patients, pharmacists, or healthcare providers-know this: the generic drug you pick up at the pharmacy might have changed its manufacturing process. But if it’s still on the shelf, the FDA has approved it. The system isn’t perfect, but it’s designed to keep you safe-even if it’s slow.


Pranab Daulagupu
November 29, 2025 AT 11:09Switching API suppliers without a PAS? That’s a one-way ticket to a warning letter. Saw a colleague get burned last year-6 months of downtime, $400K gone. FDA doesn’t play. Always assume PAS unless you’ve got 10 years of stability data backing you.
Barbara McClelland
December 1, 2025 AT 09:18Love this breakdown! 🙌 Seriously, so many small pharma folks don’t realize they can use QbD to avoid PAS hell. I’ve seen teams cut review time by half just by mapping critical process parameters early. It’s not magic-it’s just planning ahead. You got this!
Alexander Levin
December 1, 2025 AT 17:55FDA’s just protecting Big Pharma’s profits. 😏 Why else would they make it take 14 months to change a tablet press? They want you stuck with outdated, cheap tech so the brand-name guys don’t lose market share. #Conspiracy
Ady Young
December 3, 2025 AT 12:05Actually, I think the real issue isn’t the FDA being slow-it’s that most companies treat compliance like an afterthought. We started doing quarterly internal audits and pre-submission checklists after our last PAS got bounced. Cut our revision cycles by 60%. It’s not about more paperwork-it’s about smarter paperwork.
Travis Freeman
December 5, 2025 AT 01:59As someone who’s worked in both India and Ohio, let me tell you-facility transfers aren’t just about equipment. It’s the water quality, the humidity, even the way the operators breathe. We had a batch fail because the new line had a slightly different vibration pattern. FDA caught it. We fixed it. Took 11 months. Worth it.
Sean Slevin
December 5, 2025 AT 16:15Wait-so if you change a binder… you need NEW CLINICAL DATA?!?!?!?!? That’s insane. A binder isn’t the active ingredient! It’s like saying if you change the color of the car’s interior, you need to retest the engine. The FDA’s stuck in the 1980s. This system is broken. Broken. BROKEN.
Chris Taylor
December 6, 2025 AT 10:19My buddy’s company got a CBE-30 approved for a packaging change-switched from glass to HDPE. Took 3 weeks. But then they added a new foil seal and got a complete response letter. Turns out the seal affected moisture ingress. Lesson? Even tiny changes can blow up. Always test.
Melissa Michaels
December 6, 2025 AT 13:27For manufacturers considering a change: consult the FDA’s 2011 supplement guidance document. It’s publicly available. Also, ensure your analytical methods are validated under ICH Q2. Many PAS rejections stem from inadequate method transfer data. Don’t skip the basics.
Nathan Brown
December 7, 2025 AT 16:01It’s funny how we call this "regulation" but it’s really just risk aversion dressed in lab coats. The FDA’s job isn’t to optimize efficiency-it’s to prevent any possible harm, even if the chance is 0.001%. That’s noble. But it’s also why generics are disappearing from the market. We’re punishing innovation with bureaucracy.
Matthew Stanford
December 9, 2025 AT 14:50For small teams: don’t wait for the FDA to ask for something. Proactively submit a pre-submission package with your stability data, comparability protocol, and process flow. It costs nothing but time-and that time saves you months later. We did it for our PAT upgrade. Got feedback in 4 weeks. Approved in 7 months.
Olivia Currie
December 9, 2025 AT 15:59OMG I JUST REALIZED-this whole system is why we have drug shortages! 😱 One plant shuts down in Europe, the FDA takes 18 months to approve a US replacement, and suddenly insulin is rationed. We’re not just talking paperwork-we’re talking lives. This needs to change NOW.
Curtis Ryan
December 10, 2025 AT 21:33Just got my PAS approved after 14 months. Changed the tablet press. Same model. Same specs. FDA wanted 12 new batch records, 3 new validation protocols, and a 100-page comparison of vibration frequencies. I cried. Then I celebrated. Now I’m upgrading again… and I’m not telling anyone until it’s done. 😅