Pharmaceutical Patents: How They Shape Drug Prices, Access, and Innovation
When you hear pharmaceutical patents, legal protections that give drug companies exclusive rights to sell a new medicine for a set time. Also known as drug patents, they're the reason some pills cost hundreds of dollars while others cost pennies. These patents aren't just paperwork—they're the engine behind how drugs get made, priced, and who gets to use them.
Every new drug starts with a patent, usually lasting 20 years from the date it's filed. But here's the catch: by the time the FDA approves it, half that time is often already gone. That leaves companies with just 7–10 years to make back their R&D costs before generics can enter the market. That’s why brand-name drugs are expensive—they need to pay for failed experiments, clinical trials, and marketing in a short window. Meanwhile, generic drugs, cheaper versions of brand-name medicines that become available after patent expiration. Also known as off-patent drugs, they are often 80–90% cheaper because they don’t repeat expensive studies—they just prove they work the same way. And when patents expire, prices drop fast. Look at drugs like Lipitor or Humira: once generics arrived, millions saved thousands annually.
But patents aren’t just about expiration dates. Companies sometimes stretch them with minor changes—new dosages, delivery methods, or combinations—called "evergreening." This delays generics and keeps prices high. The FDA tracks these changes closely, especially when they involve FDA approval, the process that verifies a drug is safe and effective before it hits the market. Also known as drug authorization, it for generics, which must meet strict standards but don’t need to redo costly trials. That’s why manufacturing changes, like switching suppliers or tweaking production lines, can trigger FDA reviews and delays, as seen in posts about ANDA supplements and CMC changes.
And then there’s the global angle. The U.S. pays far more for brand-name drugs than other countries, partly because patents aren’t enforced the same way abroad. In places like Canada or India, patents expire earlier or are ignored for life-saving drugs. That’s why many people turn to international pharmacies—but that also opens the door to counterfeit meds. Knowing how patents work helps you spot when a drug should be cheaper, or why it’s still expensive when it shouldn’t be.
When a patent runs out, it doesn’t just lower prices—it changes everything. It affects drug shortages, insurance coverage, and even which medications doctors prescribe. You’ll find posts here about how patent cliffs cause sudden gaps in supply, how generic manufacturers scramble to fill demand, and why some patients get stuck with expensive brand-name drugs long after cheaper options exist. You’ll also see how media misrepresents generics, how double-dosing risks rise when patients switch brands, and how drug pricing ties directly to patent rules.
Understanding pharmaceutical patents isn’t about legal jargon—it’s about knowing why your prescription costs what it does, when a cheaper version might arrive, and how to ask the right questions when something doesn’t add up. Below, you’ll find real-world examples from doctors, patients, and pharmacists who’ve seen how these rules play out on the ground—no theory, just what actually happens when patents collide with real health needs.
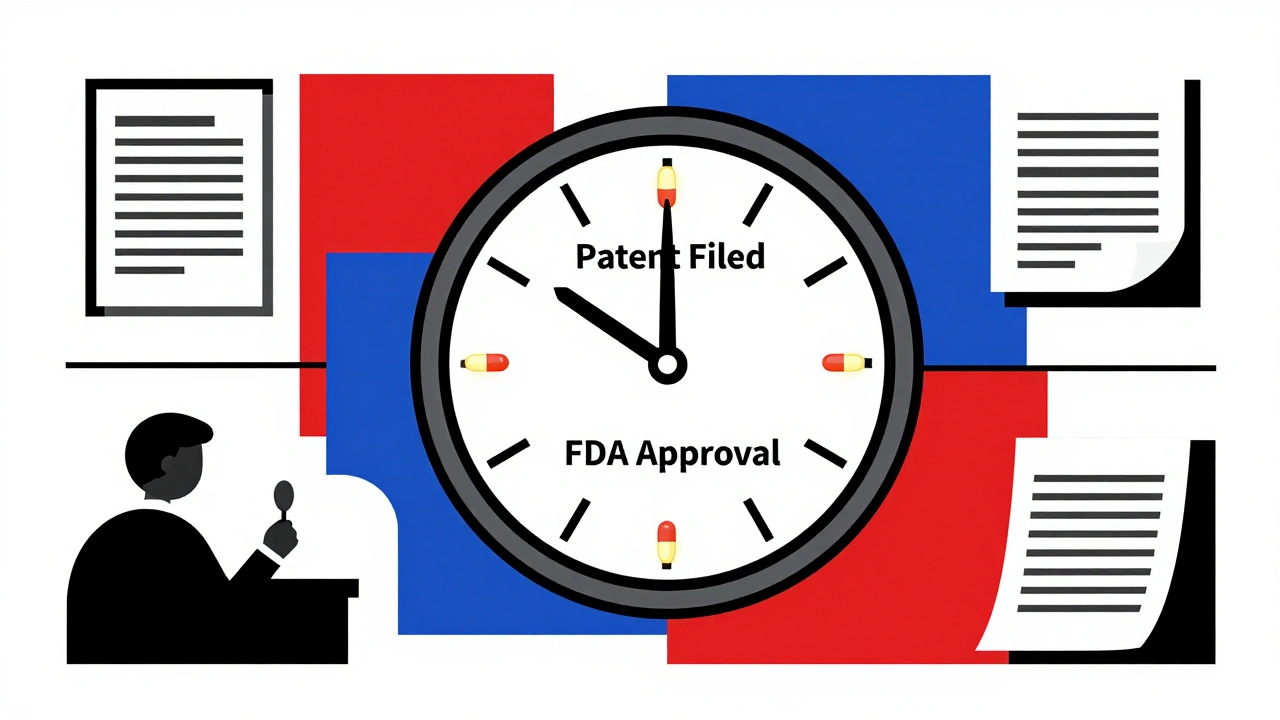
Effective Patent Life: Why Market Exclusivity for Drugs Is Shorter Than You Think
Dec 1 2025 / MedicationsEffective patent life for drugs is often just 10-13 years, not 20, due to long approval times. Secondary patents and regulatory exclusivities extend exclusivity beyond the original patent, creating complex market dynamics.
VIEW MORE