The Orange Book isn’t a book you read for fun-it’s the single most important reference pharmacists, doctors, and insurers use every day to decide whether a generic drug can safely replace a brand-name one. Published by the U.S. Food and Drug Administration (FDA), it’s officially called Approved Drug Products with Therapeutic Equivalence Evaluations. But everyone calls it the Orange Book because of its bright orange cover. First published in 1980, it was created to solve a real problem: how to let cheaper generic drugs enter the market without risking patient safety.
What Makes a Generic Drug Safe to Substitute?
Not all generic drugs are the same. Just because a pill looks like the brand-name version doesn’t mean it works the same way in your body. The Orange Book answers this question with a strict definition: therapeutic equivalence. For two drugs to be considered therapeutically equivalent, they must meet three key criteria.
First, they must be pharmaceutically equivalent. That means identical active ingredients, same strength, same dosage form-like a tablet or capsule-and same route of administration, such as oral or injectable. If one is a tablet and the other is a liquid, they’re not equivalent, no matter how similar the ingredients.
Second, they must be bioequivalent. This is the trickiest part. Bioequivalence means the generic drug gets into your bloodstream at the same rate and to the same extent as the brand-name drug. The FDA tests this using blood samples from healthy volunteers. If the generic’s absorption profile falls within 80-125% of the brand’s, it passes. That’s not a guess-it’s a scientifically proven range that ensures no meaningful difference in how the drug affects your body.
Third, the FDA must officially recognize it. The drug must be approved under the proper regulatory pathway, meet quality standards, and be labeled correctly. Only then does it earn a place in the Orange Book.
How the TE Code System Works
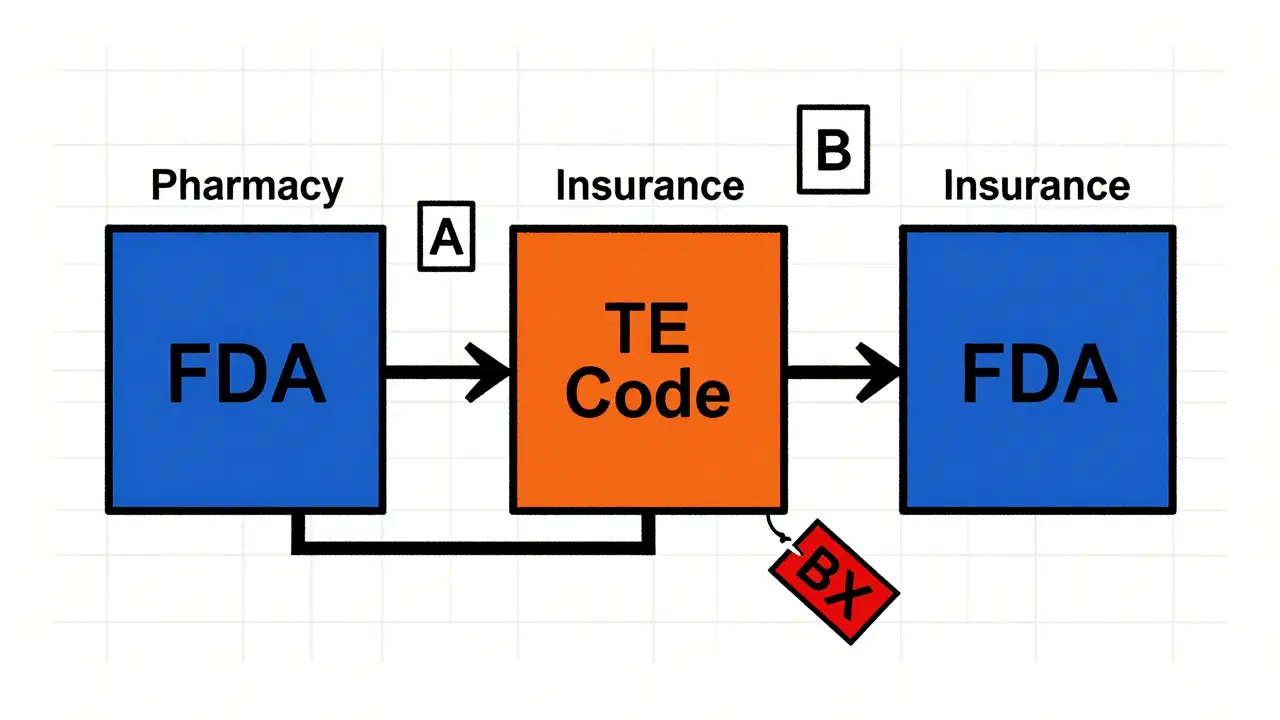
The Orange Book doesn’t just list drugs-it assigns each one a Therapeutic Equivalence (TE) code. These two-letter codes tell you at a glance whether substitution is allowed.
Codes starting with ‘A’ mean the drug is therapeutically equivalent. The second letter gives more detail. ‘AB’ is the gold standard: the generic has been proven bioequivalent with no known issues. ‘AN’ means it’s an inhalation product with bioequivalence established through non-clinical testing. ‘AO’ is for oral solutions where bioequivalence was shown using pharmacokinetic data. These distinctions matter because not all drugs are simple pills.
Then there are the ‘B’ codes. ‘BC’ means the drug has potential bioequivalence problems-maybe the formulation is complex, or the testing data is incomplete. ‘BX’ means the FDA has reviewed it and found insufficient evidence to call it equivalent. Pharmacists are legally prohibited from substituting ‘BX’ drugs without a doctor’s explicit permission.
These codes directly impact your prescription. If your insurance plan or state law requires generic substitution, the pharmacy’s system checks the Orange Book’s TE code before dispensing. A mismatched code can trigger a claim denial, delay your medication, or even lead to an incorrect drug being given.
Who Uses the Orange Book-and Why?
It’s not just pharmacists. Pharmacy benefit managers (PBMs) use it to build formularies. Insurance companies rely on it to determine which generics they’ll cover. State boards of pharmacy use it to write substitution laws. In fact, every U.S. state requires TE code verification before allowing a generic substitution.
For patients, this system saves money. Generic drugs make up 90% of all prescriptions filled in the U.S. but only cost 23% of what brand-name drugs do. Over the past decade, they’ve saved the healthcare system more than $1.6 trillion. That’s not a rounding error-it’s life-changing for people who need chronic medications like blood pressure pills, diabetes drugs, or antidepressants.
But the system isn’t perfect. Complex drugs-like inhalers, topical creams, or injectables-are harder to test for bioequivalence. A generic inhaler might have the same active ingredient, but if the device that delivers it is slightly different, the drug might not reach your lungs the same way. The FDA has issued guidance to clarify these cases, but confusion still exists. In 2022, a survey found that 67% of pharmacists found TE codes ‘moderately to extremely difficult’ to interpret without training.
Real-World Problems and Solutions
When TE codes are misread, people suffer. In Q1 2022, Walgreens reported $1.2 million in rejected claims across its stores due to incorrect substitution attempts-mostly involving ‘BC’ and ‘BD’ coded drugs. Patients got delays. Pharmacies lost money. Insurance companies flagged errors.
But some companies fixed it. CVS Health built an automated system in 2021 that checks TE codes in real time when a prescription is processed. The result? A 63% drop in substitution errors and $47 million saved annually. That’s not just efficiency-it’s patient safety.
The FDA is also modernizing. The old printed version is gone. Today, the Orange Book is a searchable online database updated monthly. The new version, rolling out fully in 2024, will show application numbers, manufacturer names, and drug strengths all in one place. It’s faster, clearer, and easier to use.
What’s Not in the Orange Book?
It’s important to know what the Orange Book doesn’t cover. It doesn’t compare different drugs used for the same condition. For example, it won’t tell you whether morphine and meperidine are interchangeable for pain. They’re different drugs, even if they treat the same symptom. That’s a clinical decision, not a substitution one.
It also doesn’t include drugs approved only for safety, like some older medications reviewed under the DESI program. And it doesn’t cover biologics-those complex protein-based drugs like insulin or Humira. Those have their own pathway and aren’t listed in the Orange Book, though biosimilars are expected to grow significantly by 2028.
How to Use the Orange Book Correctly
If you’re a patient, you don’t need to use it directly. But if you’re a pharmacist, nurse, or pharmacy student, here’s how to get it right:
- Always check the TE code before substituting. Don’t assume because it’s generic, it’s interchangeable.
- Use the FDA’s free online database. Don’t rely on memory or outdated printouts.
- When in doubt, consult the prescriber. Especially for narrow therapeutic index drugs like warfarin or levothyroxine-tiny differences can cause serious side effects.
- Stay updated. The Orange Book changes every month. New generics get added. Codes get updated. Outdated info is dangerous.
Professional training helps. The National Community Pharmacists Association offers a 4-hour certification course. Over 8,000 pharmacists took it in 2022. But even with training, only 41% of community pharmacists felt ‘very confident’ in their ability to interpret TE codes without checking the database.
The Bigger Picture
The Orange Book is the backbone of affordable medicine in the U.S. It’s what lets a person on a fixed income get their cholesterol pill for $4 instead of $400. It’s what allows hospitals to stretch their budgets without compromising care. It’s what lets insurance companies cover more drugs without raising premiums.
But it only works if people understand it. Misinterpretation leads to delays, errors, and sometimes harm. The FDA knows this. That’s why they keep improving it. The next generation of the Orange Book will be smarter, faster, and more transparent.
For now, the message is simple: don’t guess. Check the code. Use the database. When in doubt, ask. Because behind every TE code is a patient who depends on it.
What does an 'AB' code mean in the Orange Book?
An 'AB' code means the generic drug is therapeutically equivalent to the brand-name drug. It has been proven to be pharmaceutically equivalent and bioequivalent, with no known or potential bioequivalence problems. Pharmacists can substitute it without needing a doctor’s approval.
Can any generic drug be substituted for a brand-name drug?
No. Only drugs with an 'A' code in the Orange Book can be automatically substituted. Drugs with 'B' codes, like 'BC' or 'BX,' have unresolved bioequivalence issues or insufficient data. Substituting these requires a doctor’s explicit instruction.
Is the Orange Book only for prescription drugs?
No. The Orange Book includes both prescription and over-the-counter (OTC) drugs. Part I covers prescription drugs with therapeutic equivalence evaluations. Part II includes OTC drugs not covered under standard monographs. But it does not include biologics, vaccines, or drugs approved only on safety grounds.
How often is the Orange Book updated?
The Orange Book is updated monthly. New generic approvals, withdrawn products, and revised TE codes are added each month. The FDA releases the updated version online by the end of each month, so using outdated print versions is risky.
Why are some drugs not listed in the Orange Book?
Drugs approved only for safety (like some pre-1984 drugs reviewed under DESI), biologics, and drugs not approved under the ANDA pathway are not listed. Also, drugs for export, military use, or those discontinued for reasons other than safety or efficacy are excluded. The Orange Book only includes drugs approved as safe, effective, and pharmaceutically equivalent.





Candice Hartley
January 27, 2026 AT 21:21Love that the Orange Book saves people money. My dad takes warfarin and we used to pay $300/month-now it’s $12. Life-changing.
Anjula Jyala
January 28, 2026 AT 11:36TE codes are a mess nobody trains for properly. AB means nothing if the pharmacist doesn’t know the difference between bioequivalence and pharmaceutical equivalence. Pharma companies game the system with microformulation tweaks. It’s all smoke and mirrors.
John O'Brien
January 29, 2026 AT 04:47Bro the Orange Book is literally the reason I can afford my antidepressants. I’m not gonna complain about some code system that keeps me alive while saving me $400 a month. Stop overcomplicating it.
Patrick Merrell
January 30, 2026 AT 04:06They don’t tell you this but the FDA lets generics slip through with 79% bioequivalence if the manufacturer pays enough in lobbying fees. The 80-125% range? A joke. I’ve seen people crash on generics that "passed".
Marian Gilan
January 31, 2026 AT 08:45remember when they switched my levothyroxine to a generic and i started having heart palpitations? they said "it’s bioequivalent" but my body knew better. they don’t test for how you feel, only how much is in your blood. that’s not medicine, that’s math.
Kegan Powell
February 2, 2026 AT 07:07Real talk: if you’re a pharmacist or student, bookmark the FDA’s online Orange Book. Don’t trust printouts or apps that haven’t updated since 2020. I’ve seen people get the wrong drug because someone used an old PDF. It’s free. It’s updated monthly. Use it. Your patient’s life might depend on it.
Conor Flannelly
February 3, 2026 AT 20:35What’s wild is how the Orange Book mirrors the tension between efficiency and safety in healthcare. We want cheaper meds, yes-but at what cost to nuance? A pill that meets 80-125% bioequivalence might be statistically identical, but human bodies aren’t lab rats. Some of us metabolize differently. Some of us have conditions that make even 5% variation dangerous. The system works for most, but it fails the vulnerable. And we pretend that’s acceptable because the numbers look good on a spreadsheet.
It’s not just about codes. It’s about who gets to be considered "normal" in clinical testing. Healthy volunteers. Young adults. No elderly, no pregnant, no chronic kidney disease. We generalize across populations that don’t exist in reality. And then we wonder why some people say "the generic didn’t work for me."
Maybe the answer isn’t more codes. Maybe it’s more listening. More individualized data. More transparency about what "equivalent" really means when your body is the lab.
I’m not against generics. I’m against pretending we’ve solved a biological problem with a bureaucratic one.
Conor Murphy
February 4, 2026 AT 21:13Conor here. I’ve worked in Irish pharmacies for 18 years. We don’t have an Orange Book, but we have similar rules. The thing is, patients don’t care about TE codes. They care if their anxiety came back after switching. Or if their seizure frequency changed. We don’t have the time to explain bioequivalence percentages. So we default to brand unless forced. Because trust matters more than cost.
And yeah, some generics are fine. But others? You can taste the difference in the fillers. I’ve had patients say "this one tastes chalky" and they’re always right. The FDA doesn’t test taste. But bodies do.
Desaundrea Morton-Pusey
February 6, 2026 AT 14:56USA is the only country that lets corporations dictate medicine. The Orange Book is just Big Pharma’s way of pretending generics are safe while they still charge $400 for the brand. Wake up. This is capitalism, not healthcare.
Kathy McDaniel
February 7, 2026 AT 15:50i just found out my insurance won’t cover my generic because the te code was bc and they thought it was bx 😭 i had to call my dr at 8pm to fix it. why is this so hard??
Murphy Game
February 8, 2026 AT 15:32Did you know the FDA’s database is run by contractors who get paid per update? That’s why it’s always late. And why some codes disappear for weeks. They’re not trying to help you. They’re trying to hit their KPIs. The Orange Book is a rigged game.