Every year, millions of prescription drugs move through a complex network of manufacturers, wholesalers, and pharmacies before reaching your medicine cabinet. But what if one of those pills wasn’t made by the company listed on the label? What if it was fake, contaminated, or stolen? That’s not a hypothetical. It’s a real threat-and the DSCSA track-and-trace system was built to stop it.
What Is the DSCSA, and Why Does It Matter?
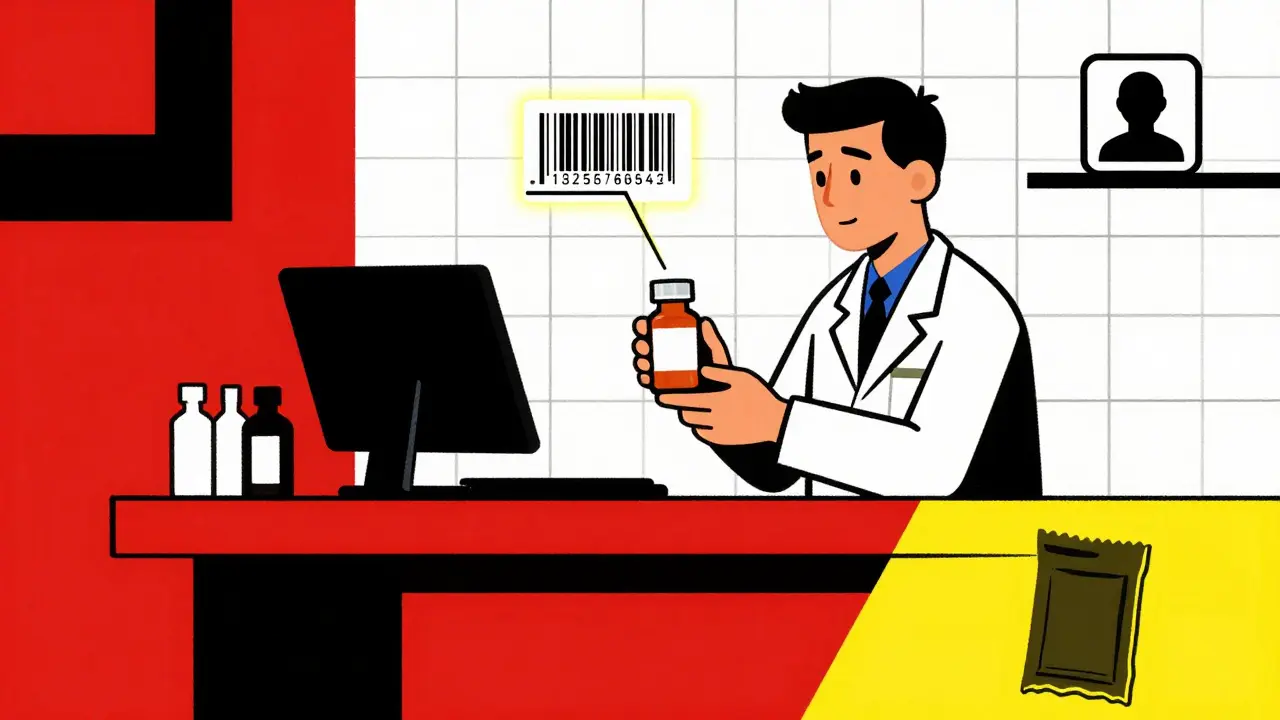
The Drug Supply Chain Security Act (DSCSA) is a federal law passed in 2013 to protect patients from counterfeit, stolen, or unsafe drugs. Before DSCSA, there was no national standard for tracking prescription medications. Each state had its own rules-or none at all. That meant a drug could be shipped across the country with no one knowing where it had been, who handled it, or whether it was real. DSCSA changed that. It created a single, nationwide system that requires every package of prescription medicine to have a unique digital fingerprint. That fingerprint includes the National Drug Code (NDC), the lot number, the expiration date, and a one-of-a-kind serial number. It’s printed on the package in both human-readable text and a machine-scannable barcode. By November 27, 2024, every pharmacy, wholesaler, and manufacturer in the U.S. had to be able to electronically verify any drug package in seconds. If a package doesn’t match the manufacturer’s record, it’s flagged as suspect. That’s how fake drugs get caught before they hit the shelf.How the System Works: The Three Keys
The DSCSA doesn’t just track packages-it verifies them at every step. Here’s how:- Transaction Information (TI): This tells you what the product is, who made it, and when it was shipped.
- Transaction History (TH): This shows the full chain of custody-every company that handled the drug before it got to you.
- Transaction Statement (TS): This is a legal certification that the drug wasn’t stolen or tampered with.
Why This System Beats Old-Style Tracking
Before DSCSA, if a drug was recalled, companies had to pull entire batches-sometimes thousands of boxes-even if only one was fake. That wasted money, disrupted care, and left patients without needed meds. Now, with serial numbers, they can pinpoint the exact package. One bottle. One box. That’s it. That’s how DSCSA reduces waste and keeps safe drugs in circulation. It also cuts down on drug diversion. That’s when someone steals medication and sells it illegally. In 2020, the FDA estimated that DSCSA’s tracking system reduced diversion by 40% in just five years. That’s tens of thousands of pills kept out of the black market. And it’s not just about theft. Counterfeit drugs are a growing problem. Fake versions of popular medications like insulin, blood thinners, and cancer treatments have been found in the U.S. supply chain. These fakes can be deadly. DSCSA makes it nearly impossible for them to slip through unnoticed.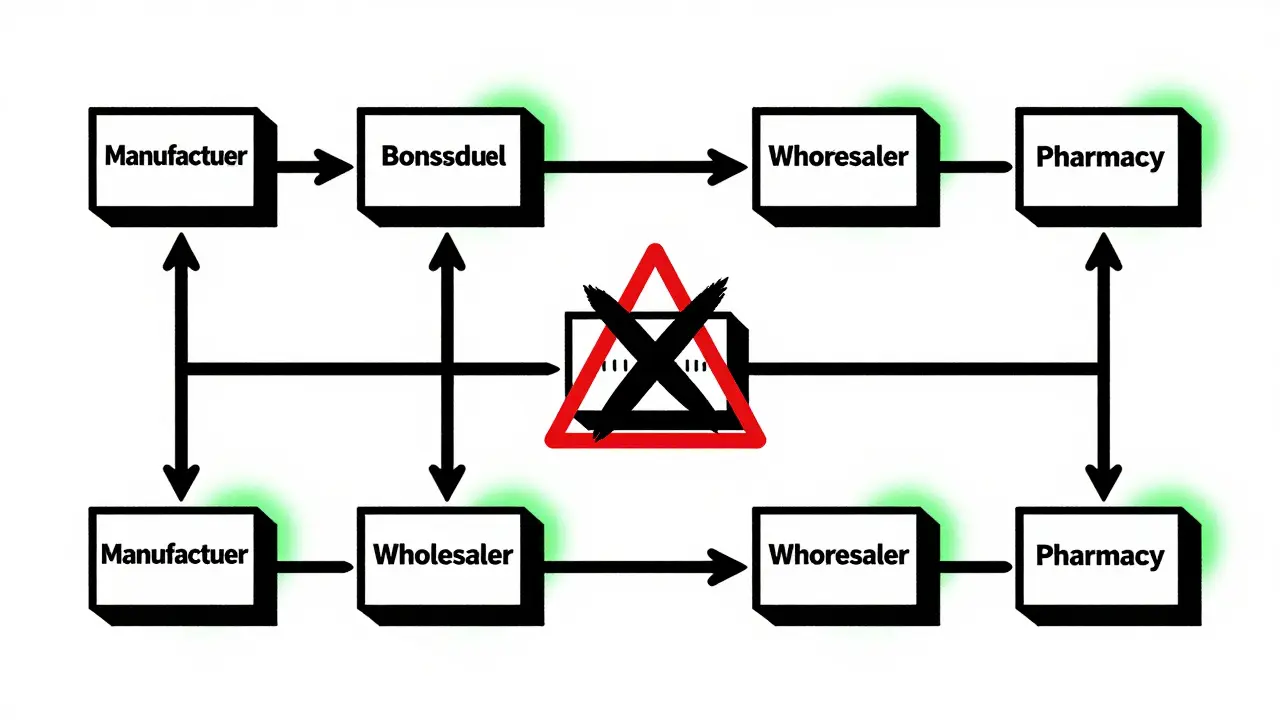
Who Has to Follow the Rules?
DSCSA applies to everyone in the chain:- Manufacturers: Must serialize every package and share data electronically.
- Repackagers: If a company repackages drugs (like turning a 100-pill bottle into 30-pill blister packs), they must also serialize and verify.
- Wholesalers: Must verify every shipment they receive and pass along transaction data.
- Dispensers: That means pharmacies-big chains and small independents alike-must scan and verify every prescription before giving it to a patient.
The Real-World Impact: Numbers That Matter
The results are clear:- 98% of manufacturers and 95% of wholesalers are fully compliant as of mid-2023.
- Only 72% of pharmacies are fully compliant-big chains like CVS and Walgreens are ahead, but many small independents are still struggling.
- CVS Health reported a 75% drop in suspect product investigations after implementing automated DSCSA verification.
- McKesson, one of the largest distributors, processed over 1.2 billion serialized transactions in 2023 with 99.98% accuracy.
- Independent pharmacies report average compliance costs of $185,000 per location, a major burden for small businesses.
What Happens If a Fake Drug Is Found?
If a pharmacy scans a package and the system says it’s not legitimate, they can’t just ignore it. The law requires them to:- Immediately stop dispensing the product.
- Quarantine it in a secure location.
- Notify the manufacturer and the FDA within 24 hours.
- Conduct an investigation to find out how it got there.
Challenges and the Road Ahead
The biggest problem isn’t the law-it’s the technology. Not every vendor uses the same software. Not every system speaks the same language. That’s why interoperability is still a challenge. The FDA’s 2023 report showed that data mismatches between trading partners remain the top reason for verification delays. Some pharmacies still rely on manual entry or outdated scanners. That’s why the FDA gave a one-year grace period after the November 2024 deadline-to let systems stabilize. But the future is coming fast. The FDA is already looking at expanding DSCSA to over-the-counter drugs-especially high-risk ones like painkillers and allergy meds. That could mean every bottle of ibuprofen you buy will soon have a serial number too. And the cost? It’s high. But the alternative is worse. A single counterfeit heart medication could kill someone. DSCSA’s cost-$100,000 to $500,000 per pharmacy-is a small price to pay to prevent that.What This Means for You
You don’t need to scan your own pills. You don’t need to check serial numbers. That’s the pharmacy’s job. But you should know this: the medicine you take today is safer than ever because of a system designed to catch fakes before they reach you. Every time your pharmacist scans your prescription, they’re not just filling a bottle-they’re protecting your life. If you’ve ever worried about fake drugs, especially after hearing stories online, know this: the system is working. It’s not perfect, but it’s the most advanced drug safety net in U.S. history. And it’s not slowing down. As technology improves, the DSCSA will get smarter. Faster. More accurate. The goal isn’t just to catch counterfeit drugs-it’s to make them impossible to distribute.What does DSCSA stand for?
DSCSA stands for the Drug Supply Chain Security Act. It’s a U.S. federal law passed in 2013 to build a secure, electronic system for tracking prescription drugs from manufacturer to pharmacy, with the goal of preventing counterfeit, stolen, or unsafe drugs from reaching patients.
When did DSCSA become fully active?
The full DSCSA track-and-trace system became mandatory on November 27, 2024. That’s when every manufacturer, wholesaler, and dispenser in the U.S. was required to exchange electronic transaction data and verify each prescription drug package at the unit level using unique serial numbers.
How does DSCSA stop counterfeit drugs?
DSCSA stops counterfeit drugs by requiring every package to have a unique serial number tied to the manufacturer’s database. When a pharmacy receives a drug, it scans the barcode and checks the serial number against the official record. If the number doesn’t match-or if the package has been reported stolen-the system flags it immediately, preventing it from being sold to patients.
Do all pharmacies have to follow DSCSA?
Yes. All pharmacies that dispense prescription drugs in the U.S. must comply with DSCSA, whether they’re part of a national chain or a small independent store. They’re required to scan and verify every prescription package before giving it to a patient.
What happens if a pharmacy can’t verify a drug?
If a pharmacy can’t verify a drug’s serial number, they must immediately stop dispensing it, quarantine it in a secure location, and notify both the manufacturer and the FDA within 24 hours. They’re also required to investigate how the product entered their supply chain. Failure to do so can result in FDA enforcement actions, including fines or shutdowns.
Is DSCSA the same as the EU’s FMD?
No. The EU’s Falsified Medicines Directive (FMD) requires physical anti-tampering seals and a central database that stores all serial numbers. DSCSA doesn’t require tamper-proof packaging or a central repository. Instead, it relies on electronic data exchange between trading partners-each company keeps its own records, but they must be able to verify each other’s data.
Will DSCSA cover over-the-counter drugs too?
The FDA is currently evaluating whether to extend DSCSA requirements to certain high-risk over-the-counter (OTC) medications, such as pain relievers and allergy drugs. While no official decision has been made, Commissioner Robert Califf has indicated this expansion is under active review as part of long-term safety planning.



Raja P
December 23, 2025 AT 22:02This is actually kind of a big deal, you know? I work in pharma logistics in India, and seeing this system roll out in the US feels like a blueprint for what we need to build back home. No more shady suppliers slipping through the cracks. Seriously, if this works, it could save lives globally.
Joseph Manuel
December 25, 2025 AT 18:01The claim that counterfeit drugs have been reduced by 95% since 2019 is statistically dubious without citing the baseline. The FDA’s own 2022 report noted only 1,200 confirmed counterfeit incidents nationwide - a fraction of total prescriptions filled. Correlation does not equal causation, and the 72% pharmacy compliance rate suggests systemic gaps. This isn’t a victory lap - it’s a halfway mark with major enforcement risks.
Andy Grace
December 26, 2025 AT 00:39Interesting read. I’ve seen this play out in Australia with the PBS system - serialization helps, but the real headache is when vendors use different data formats. One pharmacy I worked with had to manually reconcile 300 packages last month because the wholesaler’s barcode didn’t match the manufacturer’s encoding. It’s not perfect, but it’s miles ahead of what we had before.
Delilah Rose
December 27, 2025 AT 13:44I just want to say how deeply moved I am by the fact that every time my pharmacist scans my prescription, they’re not just doing their job - they’re literally standing between me and a deadly fake pill. I used to worry about this stuff after reading those scary stories on Facebook, but now I know there’s this whole invisible system working behind the scenes, like a silent guardian angel made of barcodes and databases. And even though it’s not perfect - and yeah, small pharmacies are getting crushed by the cost - at least we’re trying. We’re actually trying to protect people, not just make money. That’s rare. That’s beautiful. That’s worth fighting for. I’m so proud of my country for doing this. I wish more people knew how hard this was to build.
Bret Freeman
December 29, 2025 AT 02:24Let me get this straight - we spent billions, forced every pharmacy to upgrade their entire system, and now we’re just supposed to be grateful that we’re not getting killed by fake insulin? This isn’t innovation - it’s damage control. The FDA waited until 2024 to enforce it? That’s not a deadline, that’s a disaster waiting to happen. And don’t even get me started on how many small pharmacies are going under because of this. They’re not ‘struggling’ - they’re being sacrificed so CVS can say they’re ‘compliant.’ This system isn’t saving lives - it’s just making big corporations look good while small businesses burn.
Lindsey Kidd
December 30, 2025 AT 20:27This made me cry 😭 Seriously. I’m a nurse, and I’ve seen people skip meds because they’re scared of fakes. Now they can trust their pharmacy again. Thank you to everyone who made this happen. You’re heroes. 💙
Austin LeBlanc
December 30, 2025 AT 21:21So let me get this straight - you’re telling me that a pharmacy in Ohio can’t even verify a bottle of metformin without a $200,000 tech upgrade? And you think that’s fair? Meanwhile, big pharma and Walmart are laughing all the way to the bank. This isn’t patient safety - it’s corporate extortion dressed up as regulation. The FDA should be cracking down on the manufacturers who make the systems incompatible, not punishing the front-line workers. You want to save lives? Fix the software, not the small pharmacies.
Christine Détraz
January 1, 2026 AT 08:42I’ve been in this industry for 20 years. I’ve seen the old way - paper logs, faxed invoices, no traceability. I’ve seen people die because a batch of fake blood pressure meds slipped through. This system? It’s not perfect, but it’s the first time we’ve had a real shot at stopping it. The cost is high, yes - but so is the cost of doing nothing. I’ve watched pharmacists cry when they catch a counterfeit. Now they can do it without guilt. That’s worth everything.
Pankaj Chaudhary IPS
January 3, 2026 AT 07:39In India, we still struggle with counterfeit drugs - but what’s impressive here is the institutional discipline. No exceptions. No loopholes. This is governance at its best. Other developing nations should study this model. It’s not just about technology - it’s about accountability. If every actor in the chain is legally bound to verify, corruption has nowhere to hide. This is the future of public health - transparent, traceable, and non-negotiable.
Aurora Daisy
January 4, 2026 AT 15:36Oh wow, the Americans finally figured out how to track pills. Took you long enough. In the UK, we’ve had a working system since 2019 - no one’s crying about costs because we made the vendors pay. And we don’t need 1.2 billion transactions to prove it works. Maybe if you stopped outsourcing everything to tech consultants, you’d have less drama and more results.
Paula Villete
January 6, 2026 AT 02:34So… we spent half a billion dollars to make sure no one sells you a fake Advil… but we still can’t get a decent coffee in a government building? Priorities. 😏 But seriously - if this keeps one person from dying because they took a counterfeit blood thinner? Worth it. Even if the barcode system glitches and my prescription is delayed for three days. I’ll take three days of waiting over three days in the ICU. Just… maybe next time, let’s fix the printer too?