Every year, millions of Australians and Americans pay far more than they need to for prescriptions-just because they never asked if a cheaper, equally effective version exists. Generic medications aren’t second-rate copies. They’re the exact same drug, tested and approved to work just like the brand-name version. But knowing how to find them? That’s where most people get stuck.
What Makes a Generic Medication Real?
A generic drug isn’t just a cheaper version with a different label. It has to contain the same active ingredient, in the same strength, and work the same way in your body. The FDA (and Australia’s TGA) require generics to prove they’re bioequivalent-meaning they’re absorbed into your bloodstream at the same rate and to the same extent as the brand-name drug. The acceptable range? Within 80% to 125% of the brand’s absorption. That’s not a guess. That’s science.
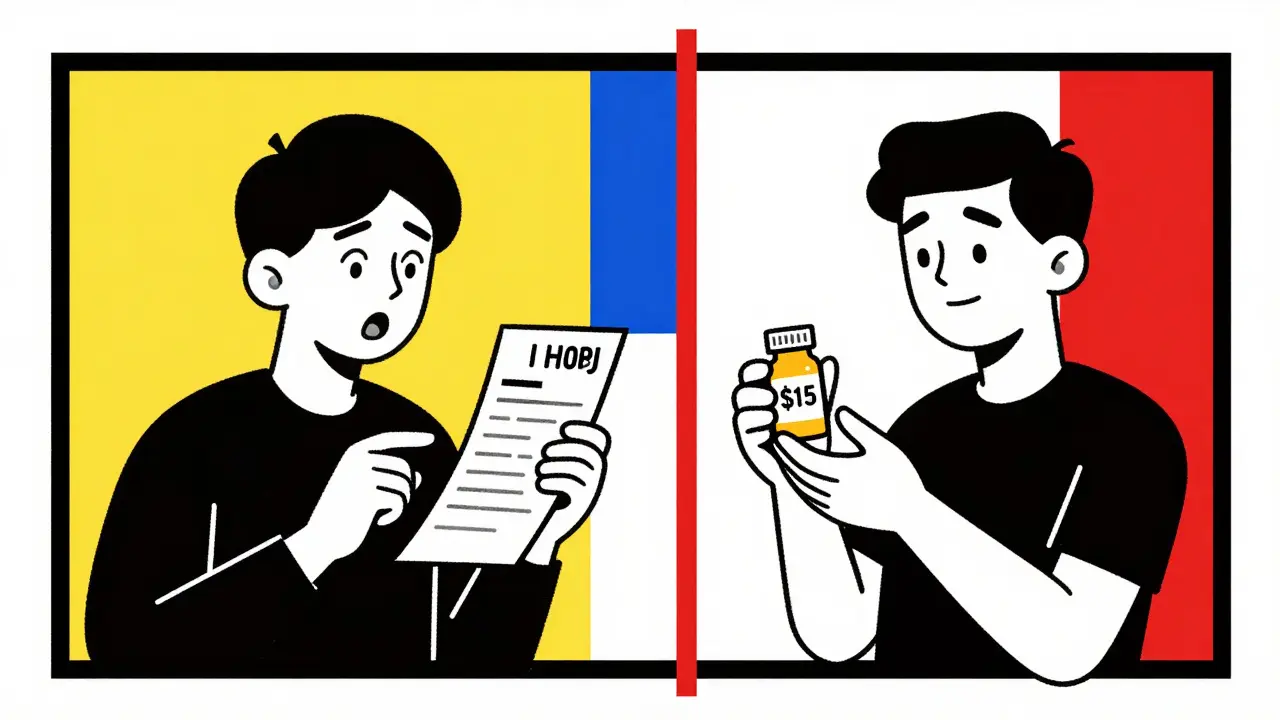
For example, if you’re prescribed Lipitor (atorvastatin), the generic version isn’t a different drug-it’s atorvastatin, made by a different company, often at a fraction of the cost. In the U.S., brand-name Lipitor cost around $765 for a 30-day supply in 2022. The generic? About $15. That’s not a savings. That’s a revolution.
Not every drug has a generic. About 10% still don’t, mostly because the patent hasn’t expired. Patents last around 17 years from filing, but with extensions and legal maneuvers, some drugs stay brand-only for over 20 years. But for the rest? There’s almost always a generic option.
How to Find Out If a Generic Exists for Your Prescription
You don’t need a medical degree to check this. You just need to know where to look-and what to ask.
1. Ask Your Pharmacist First
This is the fastest, most reliable way. Pharmacists have direct access to real-time databases like First Databank and Medi-Span. These systems flag every available generic alternative the moment your prescription comes in. A 2022 JAMA study found pharmacists correctly identify generic alternatives 98.7% of the time.
Don’t just ask, “Do you have a generic?” Say: “Is there a therapeutically equivalent generic available for this prescription?” That’s the exact phrase the American Pharmacists Association recommends. It tells them you’re not just looking for a discount-you’re looking for a medically approved substitute.
At major chains like CVS, Walgreens, and Costco, automatic generic substitution alerts are built into their systems. In 92% of cases, you’ll be offered the generic before you even leave the counter.
2. Use the FDA’s Drugs@FDA Database
If you want to check yourself, go to Drugs@FDA. Type in the brand name-say, “Zoloft.” The results will show the approved drug, the manufacturer, and the approval date.
Look for the Therapeutic Equivalence Code under the “Approval” section. You’ll see a two-letter code:
- AB = Fully equivalent. This generic can be swapped without concern.
- BX = Not rated. There’s not enough data to confirm it’s interchangeable.
- A = Bioequivalent, but formulation issues may affect interchangeability (rare).
Only AB-rated generics are guaranteed to work the same way. If you see BX, talk to your doctor before switching.
The FDA launched a simplified “Generic Drug Search” tool in October 2023. It’s easier to use than the old version and now handles over 200,000 queries a month.
3. Check Your Insurance Plan’s Formulary
If you’re on Medicare Part D, private insurance, or a government plan, your formulary lists which drugs are covered-and which generics are preferred. The Medicare Plan Finder tool updates every October 15. Log in, search your drug, and see if a generic is listed as a preferred option.
Some plans automatically switch you to the generic unless your doctor writes “Do Not Substitute.” If you didn’t ask for that, you might’ve been switched without knowing. Check your last receipt. If the name changed, that’s why your bill dropped.
4. Use GoodRx for Price Comparison
GoodRx isn’t a government tool, but it’s popular for a reason. It shows you cash prices at nearby pharmacies for both brand and generic versions. You can’t tell if a generic is AB-rated from GoodRx alone-but you can see the price difference. If the generic is 80% cheaper, it’s almost certainly an AB-rated substitute.
GoodRx has over 35 million users monthly. It doesn’t replace official sources, but it’s a great way to spot savings.
When Generics Might Not Be Safe
Most of the time, generics are perfectly safe. But there are exceptions.
Drugs with a narrow therapeutic index (NTI) need extra care. That means the difference between a helpful dose and a dangerous one is very small. Examples:
- Warfarin (blood thinner)
- Levothyroxine (thyroid hormone)
- Phenytoin (seizure medication)
- Cyclosporine (organ transplant drug)
Studies show that in 5-8% of patients taking these drugs, switching between different generic brands-even AB-rated ones-can cause changes in blood levels. That’s why doctors often recommend sticking with one brand or generic manufacturer for these medications.
If you’re on one of these drugs, don’t assume all generics are the same. Ask your pharmacist: “Is this the same manufacturer as before?” If the pill looks different, that’s normal-but if the company changed, it’s worth a quick check with your doctor.
Why You Should Always Check
In 2022, the average brand-name drug cost $765. The average generic? $15.23. That’s not a typo. That’s a 98% drop.
One Reddit user saved $1,200 a year just by asking for generics on three prescriptions. Another found their $400/month antidepressant had a $12 generic version. They’d been paying the brand price for two years.
And it’s not just about money. In 2023, 41% of Medicare beneficiaries reported confusion when their plan switched them to a generic without notice. Some thought the new pill was a mistake. Others worried it wasn’t working. It was the same drug-just cheaper.
Generic drugs account for 90% of all prescriptions filled in the U.S. But only 32% of patients understand what “therapeutic equivalence” actually means. You don’t need to be a scientist. You just need to ask.
What to Do If No Generic Is Available
If your drug has no generic yet, ask your doctor if there’s another medication in the same class that does. For example, if you’re on a brand-name statin with no generic, ask if another statin (like rosuvastatin or simvastatin) is an option. Many have generics and work similarly.
Also check the ASHP Drug Shortages database. Sometimes, even generics are unavailable because of supply chain issues. If your pharmacy says “no generic,” it might mean “none in stock,” not “none exists.”
And if you’re on a fixed income, don’t give up. Many manufacturers offer patient assistance programs. Some pharmacies have $4 generic lists. Your pharmacist can help you find them.
What’s Changing in 2024
Things are getting easier. Starting January 1, 2024, Medicare Part D plans must show real-time generic availability in the Plan Finder tool. That means you’ll know before you even fill your prescription.
By Q3 2024, Epic Systems-the electronic health record platform used by 250 million patients-will start showing FDA therapeutic equivalence codes directly in doctors’ prescription screens. That means your doctor might suggest a generic before you even leave the office.
More than 10,000 small-molecule generics are approved. Another 66 billion dollars’ worth of brand-name drugs will lose patent protection by 2028. The trend is clear: generics are the future. But you still have to ask for them.
Quick Checklist: Your Action Plan
- Look at your prescription. Write down the brand name.
- Ask your pharmacist: “Is there a therapeutically equivalent generic available?”
- If they say yes, ask: “Is it AB-rated?”
- For high-risk drugs (warfarin, thyroid meds), ask: “Is this the same manufacturer as before?”
- Use Drugs@FDA to double-check the therapeutic equivalence code.
- Compare prices with GoodRx-sometimes the generic is cheaper even without insurance.
- If your plan switched you without telling you, call them. You have the right to know.
It takes less than five minutes. The savings can be hundreds-or thousands-of dollars a year. And you’re not taking a risk. You’re just using the same medicine, at a price that makes sense.
Are generic medications as effective as brand-name drugs?
Yes. Generic medications must meet the same strict standards as brand-name drugs. They contain the same active ingredient, in the same strength, and are absorbed into the body at the same rate and extent. The FDA requires them to prove bioequivalence through testing. For most people, generics work just as well. The only exceptions are a small group of drugs with narrow therapeutic windows, like warfarin or levothyroxine, where even small changes can matter.
Why do generic pills look different from brand-name ones?
By law, generic drugs can’t look exactly like the brand-name version-this avoids trademark infringement. That’s why the shape, color, or imprint might be different. But the active ingredient, dosage, and effectiveness are identical. The differences are only in inactive ingredients like dyes or fillers, which don’t affect how the drug works.
Can I switch between different generic brands?
For most medications, yes. If the generic is rated AB by the FDA, it’s considered interchangeable. But for drugs like warfarin, thyroid meds, or seizure medications, it’s safer to stick with the same manufacturer. If you notice changes in how you feel after switching generics, talk to your doctor. Your blood levels might need checking.
Why doesn’t my pharmacy always offer a generic?
Sometimes the doctor wrote “Dispense as Written” or “Do Not Substitute” on the prescription. Other times, your insurance plan doesn’t cover the generic for that specific drug-or the pharmacy is out of stock. Always ask if a generic is available. If they say no, ask why. You have the right to know your options.
How do I know if a generic is approved in Australia?
In Australia, check the Therapeutic Goods Administration (TGA) database. Search for your brand name, then look for “Therapeutic Goods Order (TGO) 101” listings, which indicate approved generics. You can also ask your pharmacist-they have access to the same data and can confirm if a generic is approved for use here.